How do I control the temperature of my distillation?
In a pot still, you can’t.
A guy phones me one day and says the reflux column I made him doesn’t work. What do you mean? There is nothing coming out, he says. After 20 minutes of troubleshooting I discovered that he was using about 0.6kW of power to maintain his boiler at 78C which meant it was not boiling and there was no vapour going up the column. There is a very common misconception that the way to distil alcohol is by maintaining the boiler at a certain temperature around the 78-79C mark.
Please be patient with this article as there are a few concepts that need to be understood to appreciate the whole story. It will all come together near the end.
The way I explain it is by asking what temperature water boils at on the stove? 100C. Correct, if the pressure is atmospheric. What happens if you turn the dial down to get the water to simmer; what is the temperature now? 100C. Correct. So, what is the difference between water boiling hard and only simmering? It is the amount of vapour coming off the surface of the water. If you want to get technical, it the mass flow rate of vapour that is changing. What is happening is that the energy you are putting into the system is used to change the water from a liquid at 100C to a vapour at 100C. The temperature does not change, but the phase of the water changes from a liquid to steam. The energy is used to break the bonds holding the molecules in the liquid phase and release the molecure into the more excited state of vapour. This energy is called enthalpy of vapourisation (or heat of vapourisation). For water you need 2257 joules of energy to convert 1 mole of water to a vapour (1 mole of water = 18 grams).
Power is the rate of application of energy, so if you are providing 2257 joules every second, the power is 2257 watts (energy divided by time). If you provide 2257 joules in 37 seconds the power is 61 watts.
What happens when you mix water with alcohol and you add energy? (Are we getting close to talking about distillation?). Well, let’s look at the same concepts we looked at for water – what temperature does alcohol boil at? 78.3C at atmospheric pressure. And, what is the ethalpy of vapourisation of alcohol? 837 joules per mole (1 mole of alcohol = 46g). What you can see is that it takes a whole lot less energy to make alcohol turn to vapour than it does with water. What this means is that when an alcohol/water mixture boils, there will be more alcohol turning to vapour than water, which is exactly what distillation is all about.
Additionally, what all this means is that when you have alcohol and water mixed, the temperature at which the mixture boils will be between 78.3C and 100C and the actual boiling temperature will be determnied by the relative fractions of alcohol and water. The more alcohol in the mixture, the closer the temperature will be to 78.3C, and the more water, the closer the temperature will be to 100C. Exactly what these temperatures are is determined by experimentation and shown in a Vapour/Liquid Equilibrium chart (VLE chart). And what it all boils down to (sorry 🙄) is that you can’t control the temperature at which the liquid boils – it will boil at the temperature at which is boils. What you can control is the amount of vapour you produce and this is quite important for determining how long it takes to distil you product.
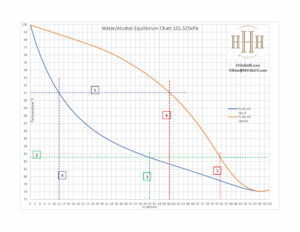
VLE example
Let us look at how to determine at what temperature our mixture will boil. Since, in distilling, we talk about fractions in terms of % alcohol by volume (ABV), the VLE chart has been produced to represent that measure. I have drawn some lines on the chart to help make the concepts clearer. Our example is of a gin distillation where the ABV in the boiler is 50%. On the VLE we have two curves, one for the liquid and one for the vapour. If we take 50% on the bottom axis and draw a line straight up (look at Line #1) until it intersects with the bottom curve (the liquid curve). From the intersection point draw a line horizontally (look at Line #2) to the side axis. You will see that the boiling temperature is between 82C and 83C. You can do this exercise for any ABV in your boiler.
You can now determine what the ABV of your vapour will be by extending Line #2 to the right until it intersects with the upper curve (the vapour curve). From the intersection point draw a line straight down to the bottom axis to determine the ABV of the vapour. In this case it is almost 80%. There is a catch to this though; the VLE curve is based on the assumption of an ideal world with no heat losses. We know that there are heat losses from out column and I have measured up to 3C temperature difference between the boiler and the top of the column in winter. In summer it will be less. If we take a temperature difference of -2C and take Line #2 and drop it from 82.5C to 80.5C (you have to do this yourself this time), the corresponding ABV of the vapour is 84%. The ABV of the vapour at the top of the column will be the same as the ABV that is measured in your parrot. You can now predict the temperature at which your mixture will boil, and the ABV in your parrot.
We have looked at an instance in time when the boiler has just started boiling and we have not collected any alcohol yet. But, over the next hours we will have collected a fair amount of alcohol and as we remove alcohol from the boiler, the ABV in boiler reduces. And what happens to the temperature as the ABV goes down? The temperature goes up! Let us continue with our gin distillation example and look at the point where we make our final cut of gin. There is still alcohol in the boiler so the temperature will not be 100C, but we know it will be less than 100C. From my expereince and the experiences shared by other distillers, a reasonable point to make the final cut would be when the temperature of the boiler is around 91C. Let’s draw a line from 91C horizontally (look at Line #5). At the intersection point with the liquid curve, draw a line vertically to the nottom axis (look at Line #4). It shows that the ABV in the boil at this time is about 12%.
Now take the intersection with Line #5 and the vapour curve, and draw a line vertically to the bottom axis. The ABV of the parrot will be about 58% in an ideal world. But, as discussed above, the temperature in the column will be about 2C less. Draw the horizontal line yourself from 89C until it intersects with the vapour curve; now draw a vertical line down from that intersection to the bottom axis and the ABV is abour 64%.
In summary, you cannot control the temperature in the boiler, but you can control the amount of vapour that you produce. As a distillation proceeds, alcohol is removed form the boiler and the temperature in the boiler will increase. Because there are heat losses in the column, the ABV at the top of the colum will be more than the ABV in the boiler.
There is a way to control the temperature at the top of the column, but it requires a different design called a reflux column. This concept will be discussed in another blog one day.

